A new article by Prof. Daniel Gryko in Angewandte Chemie International Edition
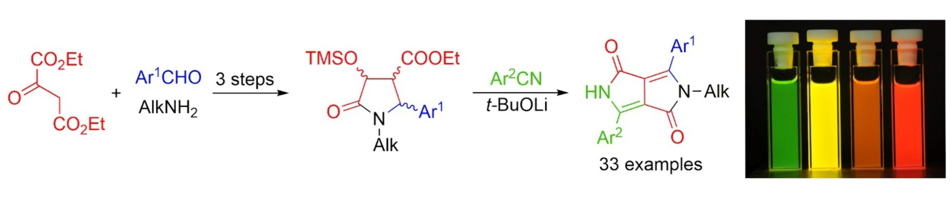
The article presents a new methodology for the synthesis of diketopyrrolopyrroles with selectively alkylated nitrogen. The presented approach uses cheap and commercially available starting materials such as: aromatic aldehydes, primary amines, aromatic nitriles and diethyl oxalacetate. For the first time very electron‐rich and sterically hindered substituents can be appended to the diketopyrrolopyrrole core by condensation of an appropriate nitrile with a pyrrolidin‐2‐one intermediate. At the same time, the methodology offers the possibility of selective introduction of C-aryl and N-alkyl substituents in a predictable manner. The variety of the obtained systems of unsymmetrically substituted diketopyrrolopyrroles significantly increases the possibility of testing the full potential of the photophysical properties of DPP structures.
The article is available at: https://onlinelibrary.wiley.com/doi/epdf/10.1002/anie.201915953