Article by Prof. Daniel Gryko in Angewandte Chemie International Edition
Prof. Daniel T. Gryko, Gana Sanil and Dr. Maciej Krzeszewski, together with colleagues from Warsaw University and from France, discovered the unprecedented tandem intramolecular hydroarylation of alkynes accompanied by a 1,2-aryl shift. Harnessing the unique electron-rich character of 1,4-dihydropyrrolo[3,2-b]pyrrole scaffold, they demonstrated that the hydroarylation of alkynes proceeds at the already occupied positions 2 and 5 leading to a 1,2-aryl shift. Remarkably, the reaction proceeds only in the presence of cationic gold catalyst, and it leads to heretofore unknown π-expanded, centrosymmetric pyrrolo[3,2-b]pyrroles. Computational studies of the reaction mechanism revealed that the formation of the six-membered rings accompanied with a 1,2-aryl shift is both kinetically and thermodynamically favorable over plausible formation of products containing 7-membered rings. The key input was provided by Prof. Witold Danikiewcz, Dr hab. Wojciech Chaładaj and Dr. Olga Staszewska-Krajewska.
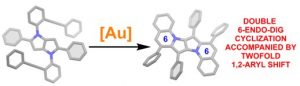
The results were published in Angewandte Chemie Int. Ed. “Gold-Catalyzed 1,2-Aryl Shift and Double Alkyne Benzannulation”: https://onlinelibrary.wiley.com/doi/10.1002/anie.202311123